All products from Protac are CE marked as medical devices and comply with the rules and requirements in the legislation under the Medical Device Regulation (MDR). At Protac, our work related to MDR goes hand in hand with our high standards of quality and is an integrated part of our quality management, ultimately enhancing patient safety.
The CE mark signifies that the production complies with applicable EU regulations, and the CE mark commits Protac to documenting the quality, safety, and performance of the products, e.g., through the development of clinical evaluations.
Protac’s products are registred in EU 2017/745 (MDR) Class 1.
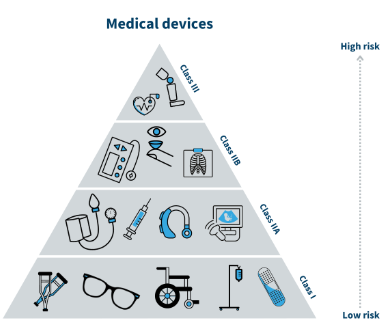
Source: Danish Medicines Agency

