Recycling the unique Protac balls
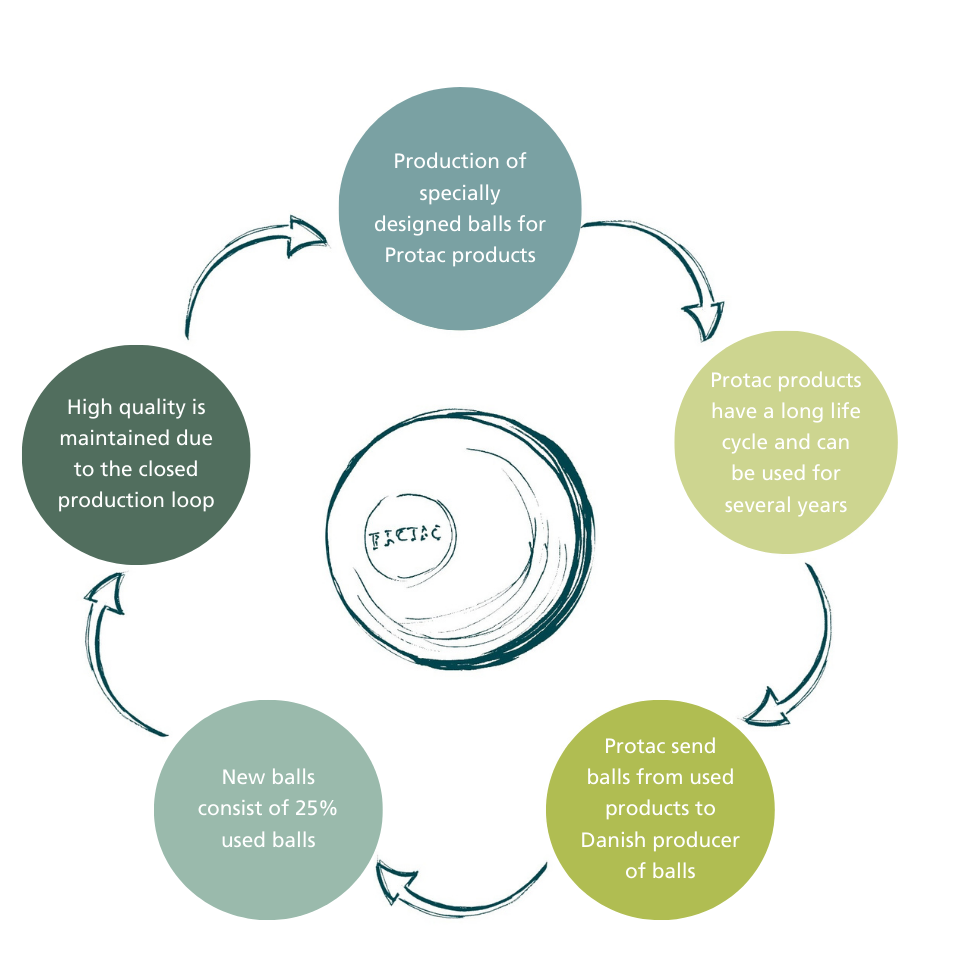
We carefully consider the materials we use and how our production impacts the environment. Since our products are CE marked and since we have high demands for the quality and hygiene of the raw material, we use, we depend on using only clean, tested, and approved plastic balls in our products. For the time being plastic is the only material that can fulfill the current high demands.This is why we have developed a recycling process in Denmark where we recycle the plastic balls from previously used products and use them to produce new Protac balls. By reusing, we ensure that the high quality of the plastic is ensured and still we are able to reduce the plastic consumption.
With this initiative, we want to make it easy for our customers to return used Protac products to us and make it possible for ourselves to reuse our own plastic of a well-known and approved quality in the production of new balls. This is a winning process for all of us.